Kentucky – A Kentucky family has filed a federal lawsuit claiming their 4-month-old daughter developed infant botulism after consuming ByHeart infant formula that was allegedly contaminated with harmful bacteria. The lawsuit, filed Wednesday in the Eastern District of Kentucky, describes the incident as “a parent’s worst nightmare.”
According to the complaint, Hanna and Michael Everett of Richmond said their baby, Piper, was “happy” and “healthy” before they began feeding her ByHeart formula, a product marketed as a cutting-edge, clinically proven alternative to traditional baby formula. The parents purchased multiple cans through Amazon after being persuaded by advertising that described the formula as superior in nutrition and quality.
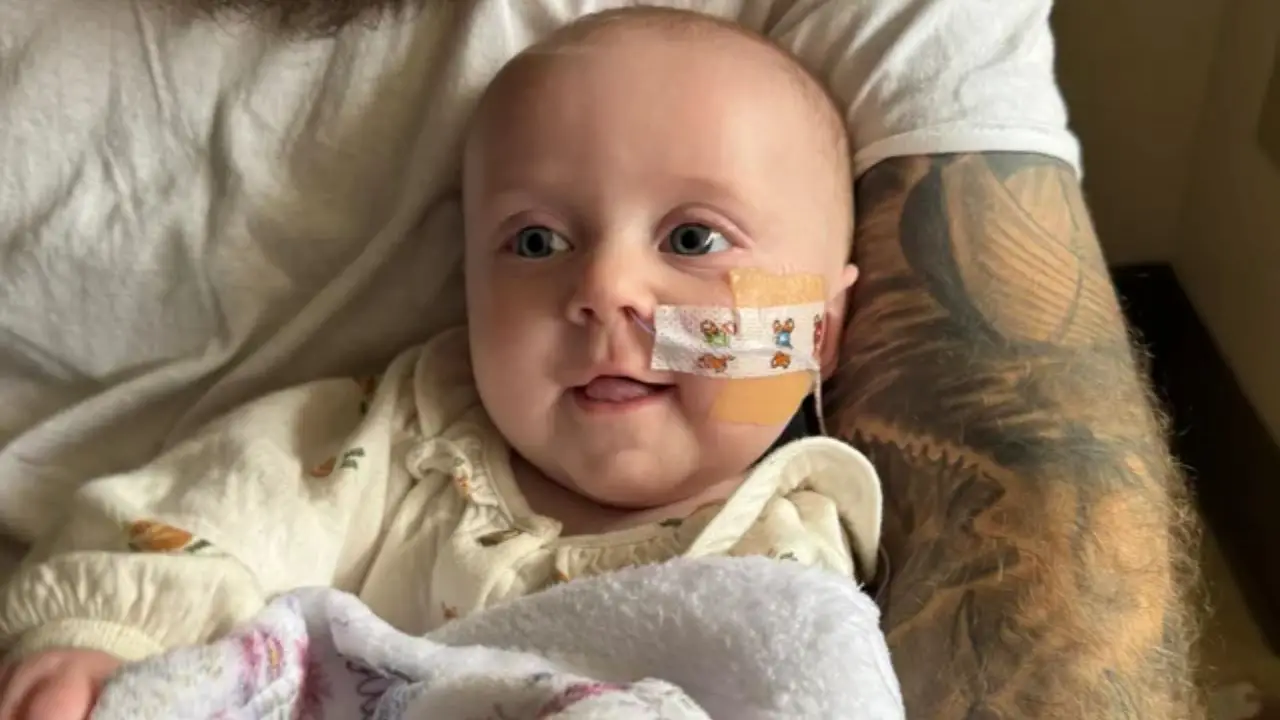
However, shortly after consuming the product, Piper reportedly began experiencing constipation, lethargy, and a series of increasingly alarming neurological symptoms. According to the lawsuit, she eventually lost her gag reflex, became unable to take a bottle, and showed signs of neurological distress that prompted an emergency trip to the hospital.
Doctors Diagnosed Infant Botulism
Piper was admitted to Kentucky Children’s Hospital on November 9, 2025, where doctors made a clinical diagnosis of infant botulism, a rare but potentially life-threatening illness caused by toxins produced by Clostridium botulinum bacteria. The condition can cause muscle weakness, feeding difficulties, respiratory problems, and in severe cases, paralysis.
The lawsuit states that physicians immediately ordered botulism antitoxin, a treatment described as “extremely scarce” and difficult to produce. The antitoxin was flown into Kentucky and successfully administered, stabilizing Piper’s condition.
Hanna Everett told the Associated Press she selected the ByHeart formula because she believed it would be “similar to breast milk.” She described the terrifying moment when she realized something was seriously wrong, saying, “I was like, ‘Oh my god, we need to go to the ER.’”
Read Also: Las Vegas-Area Road-Rage Shooting Leaves 11-Year-Old Dead on His Morning School Route
Investigation Tied Illness to Recalled Batch
The Kentucky Department of Public Health reportedly opened an investigation following Piper’s diagnosis. According to the complaint, officials determined that she had consumed formula from a batch that had been recalled due to bacterial contamination. The Everetts assert that their daughter is one of at least 15 infants in 12 states who became ill in a multistate outbreak linked to ByHeart formula.
At least one other family in Arizona has also filed suit, the complaint notes, raising concerns about whether other batches or distribution channels may have been affected. The legal filing accuses ByHeart Inc. of negligence, misleading advertising, and product failure.
The lawsuit claims that ByHeart’s branding, which emphasizes purity, quality, and advanced clinical formulation, misled parents into believing the product was safer than conventional formula options. The Everetts say they relied on those claims when choosing the brand for their daughter.
Mother Says Recovery Is Slow but Improving
Piper’s symptoms intensified over several days, the complaint says, with the infant becoming increasingly weak and unable to feed on her own. Once hospitalized, she required a feeding tube and round-the-clock monitoring.
On Facebook, Hanna Everett wrote that her daughter is “now on the upside of this” but still struggling with significant feeding challenges. She said Piper remains on a feeding tube while awaiting evaluations from speech and feeding therapists to determine when she may safely return to oral feeding.
“This never should have happened,” the mother wrote, adding that the family is grateful Piper survived the infection but is still dealing with ongoing medical complications.
Company Faces Growing Scrutiny
ByHeart, a relatively new entrant in the formula industry, markets itself as delivering premium, research-driven infant nutrition. However, the lawsuits and public health investigations have brought intense scrutiny to the company’s manufacturing and quality control practices.
Infant botulism is extremely rare, and contamination of commercially produced baby formula is even more uncommon, experts say. Public health officials have not yet disclosed whether additional recalls or warnings may be forthcoming as the investigation expands.
What Comes Next
The Everetts are seeking compensation for medical expenses, emotional distress, and what they describe as lasting harm caused by the contaminated formula. The lawsuit alleges that the company failed to properly test, screen, and monitor its product before releasing it to consumers.
With more families coming forward and state-level investigations underway, the case could become a major test for safety oversight in the infant-formula industry, which has faced heightened public concern since supply shortages and contamination recalls in recent years.
The federal court has not yet scheduled an initial hearing.
Have thoughts on this case or concerns about infant-formula safety? Share your perspective at mikeandjonpodcast.com.